2021 is the year of the Ox (丑).
This year 2021 starts at the 4351st place after the decimal point of the circular constant π.

2021 is the year of the Ox (丑).
This year 2021 starts at the 4351st place after the decimal point of the circular constant π.

Gladish, Daniel K.; Saito, Susumu; Miki, Yasushi; Niki, Teruo.
Yasushi Miki, Susumu Saito, Teruo Niki, Daniel K. Gladish
Applications in Plant Sciences, vol. 8, May 2020
https://doi.org/10.1002/aps3.11347

Figure 7
Visualization of the 3D interpretation of three late‐maturing metaxylem vessels (LMX s) and their initial cells viewed from three different angles. (A) Plan view of 3D reconstruction. (B–D) Corresponding sectional views. The pericycle and plerome are shown in translucent green and dark green colors, respectively.
Susumu Saito, Teruo Niki and Daniel K. Gladish
Plants 2020, 9, 374; https://doi.org/10.3390/plants9030374
Abstract: Root apical meristem histological organization in Zea mays has been carefully studied previously. Classical histology describes its system as having a “closed organization” and a development of xylem that conforms to predictable rules. Among the first cell types to begin differentiation are late-maturing metaxylem (LMX) vessels. As part of a larger study comparing domestic maize root development to a wild subspecies of Z. mays (teosinte), we encountered a metaxylem development abnormality in a small percentage of our specimens that begged further study, as it interrupted normal maturation of LMX. Primary root tips of young seedlings of Zea mays ssp. mexicana were fixed, embedded in appropriate resins, and sectioned for light and transmission electron microscopy. Longitudinal and serial transverse sections were analyzed using computer imaging to determine the position and timing of key xylem developmental events. We observed a severe abnormality of LMX development among 3.5% of the 227 mexicana seedlings we screened. All LMX vessel elements in these abnormal roots collapsed and probably became non-functional shortly after differentiation began. Cytoplasm and nucleoplasm in the abnormal LMX elements became condensed and subdivided into irregularly-shaped “macrovesicles” as their cell walls collapsed inward. We propose that these seedlings possibly suffered from a mutation that affected the timing of the programmed cell death (PCD) that is required to produce functional xylem vessels, such that autolysis of the cytoplasm was prematurely executed, i.e., prior to the development and lignification of secondary walls.

T. Niki, S. Saito and Y. Miki
Image processing section, mikiOn, LLC
第50回記念根研究集会(2019年11月23, 24日 名古屋)
薄切片を用いた3次元像の構築は形態観察において、重要なツールである。演者らはこれまで薄切片法の開発、薄切片による3次元像の構築を試みてきた。 ここに連続薄切片による3Dイメージの構築法を完成させたので報告する。
材料としてテオシント根先端部位を用い,ホルマリンで固定した。洗浄・脱水後テクノビット7100樹脂に包埋し、1μm厚横断・縦断の連続切片を作成した。各切片はRNA分解酵素で処理し,トルイジンブルー染色し,個々の細胞の形状・位置を特定できる高精細画像を得た(Niki et al 2019)。画像の位置合わせ、各細胞の色付けなどを行い、得た80枚の画像を重ね合わせ3次元体(3D Cuboid)を作成した。
作成された3次元体から任意方向の縦断面像・縦断面像を得ることができた。また、要素抽出によって組織構造体の3D像を作成することが可能で、切片の組織・細胞の色付けにより、根先端部の構造を明らかにできた。同一試料の横断面像、縦断面像、3D像の実現は、組織細胞形態を観察する上で有益な方法となると思われる。
It’s exactly true!
a = -80538738812075974;
b = 80435758145817515;
c = 12602123297335631;
a3 = a^3
b3 = b^3
c3 = c^3
a3 + b3 + c3
-522413599036979150280966144853653247149764362110424
520412211582497361738652718463552780369306583065875
2001387454481788542313426390100466780457779044591
42
Teruo Niki, Susumu Saito and Yasushi Miki
Image processing section, mikiOn, LLC
31st Meeting of the Japanese Society of Plant Morphology (Sendai, 9/14/2019 )
切片による2次元像、3次元像の構成には、クリヤーな画像データが大量に必要である。 この目的に、有効かつ簡易な切片作成法を開発したので報告する(Niki et al 2019)。
材料としてテオシント根先端部位を用い、ホルマリンで固定した。洗浄・脱水後テクノビット7100樹脂に包埋した。高性能ミクロトームで連続切片を作成。切片を(RNA分解)酵素で処理後、トルイジンブルー染色し、ノンカバー対物レンズ(x50、x100)を使用し観察した。
1µm切片は酵素処理によって解像性、コントラストとも満足する像を示し、ノンカバー対物レンズの使用は観察作業の効率を高めた。この手法は、3次元像等の構成を容易にした。
Susumu Saito, Teruo Niki and Daniel K. Gladish
Plants 2019, 8(6), 162; https://doi.org/10.3390/plants8060162
Classical histology describes the histological organization in Zea mays as having a “closed organization” that differs from Arabidopsis with the development of xylem conforming to predictable rules. We speculated that root apical meristem organization in a wild subspecies of Z. mays (a teosinte) would differ from a domestic sweetcorn cultivar (‘Honey Bantam’). Careful comparison could contribute to understanding how evolutionary processes and the domestication of maize have affected root development. Root tips of seedlings were prepared and sectioned for light microscopy. Most sections were treated with RNase before staining to increase contrast between the walls and cytoplasm. Longitudinal and serial transverse sections were analyzed using computer imaging to determine the position and timing of key xylem developmental events. Metaxylem development in mexicana teosinte differed from sweetcorn only in that the numbers of late-maturing metaxylem vessels in the latter are typically two-fold greater and the number of cells in the transverse section of procambium were greater in the latter, but parenchymatous cell sizes were not statistically different. Promeristems of both were nearly identical in size and organization, but did not operate quite as previously described. Mitotic activity was rare in the quiescent centers, but occasionally a synchronized pulse of mitoses was observed there. Our reinterpretation of histogen theory and procambium development should be useful for future detailed studies of regulation of development, and perhaps its evolution, in this species. View Full-Text
Keywords: metaxylem; root development; procambium; histogen; Zea mays; teosinte
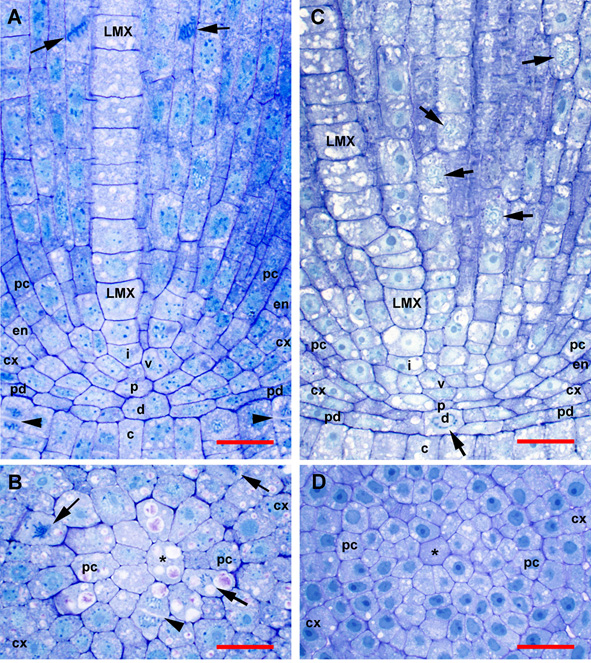
Figure 1
Organization of tissues in the promeristem and primary meristem zones of teosinte and sweetcorn roots at high magnification. Sections were not RNase treated (compare to Figure 2 and Figure 3). (A) Slightly off-median longitudinal section of teosinte. (B) Transverse section through the plerome of teosinte showing margin initial during cytokinesis. (C). Slightly off-median longitudinal section of sweetcorn. (D) Transverse section through the plerome of sweetcorn. LMX, late-maturing metaxylem vessel; asterisk (*), plerome central cell; arrow, mitotic cell; arrowhead, newly forming cell plate; c, calyptrogen; cx, immature cortex; d. dermatogen-periblem complex; en, immature endodermis; i, LMX initial cell; p, plerome; pc, pericycle; pd, protoderm; v, vascular initials layer. Scale bar = 25 µm.
T. Niki, S. Saito, and D. K. Gladish
 https://doi.org/10.1080/10520295.2019.1601769
https://doi.org/10.1080/10520295.2019.1601769
ABSTRACT
We developed a novel sectioning and staining method to make high contrast, high resolution sections of plant tissue for light microscopy. Specimens of teosinte (Zea mays L., ssp. mexicana) root tips were fixed and embedded in Technovit 7100™ plastic resin. Thin sections, 1−2.5 μm, were cut and mounted on glass slides. The sections were either treated with RNase or not, then stained with 0.1% toluidine blue O and observed through ∞/0 objective lenses. For light microscopy, the enzyme staining procedure increased resolution and contrast. High magnification ∞/0 objective lenses produced high quality images for digital photography without using a coverslip or immersion oil. Our slide preparation and microscopic analysis were less labor intensive and more rapid than previous methods and enabled rapid and precise alignment of serial transverse sections for both tracking cell lineages and tissue measurements.